262 Balancing Chemical Equations Answer Key | 0 ratings0% found this document useful (0 votes). Which of the following rules should you follow to balance chemical. Balance and classify five types of chemical reactions: Balance the following chemical equations. Coefficient, combination, compound, decomposition, double replacement, element, molecule, product in the balancing chemical equations gizmo™, look at the floating molecules below the initial reaction:
Balance each of the following equations. Question 5 translate the following statements into chemical equations and then balance them. Balancing chemical equations name hour standard 1 learning module 10 background information and why: 1) 1 n 2 + 3 h 2 → 2 nh 3 2) 2 kcio 3 → 2 kcl + 3 o 2 3) we have tutors online 24/7 who can help you get unstuck. Write a balanced chemical equation for the following reaction :
Enter a chemical equation to balance: Hydrogen gas combines with nitrogen to form ammonia b. How to balance a chemical reaction by making sure you have the same number of atoms of each element on both sides. ⚛ reactants are written on the left hand side of the chemical equation. Which of the following rules should you follow to balance chemical. Answers in as fast as 15 minutes. Many motivators misconception about the material they provide when meeting audiences. Called subscripts are used to balance chemical equations. A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. The difference between word equations, formula equations, and chemical equation. A balanced chemical equation has an equal number of atoms of different elements in the reactants and products. Chemical equations are symbolic representations of chemical and physical changes. It represents how reactants react to give a completely new product.
This means that chemical equations need to be balanced before they can be used for calculations. When balancing a chemical equation, there are a number of steps that need to be followed. A chemical equation is the representation of total composition change related to the chemical reaction. Identify the reactants and the products in the reaction and. Synthesis, decomposition, single replacement, double replacement, and combustion.
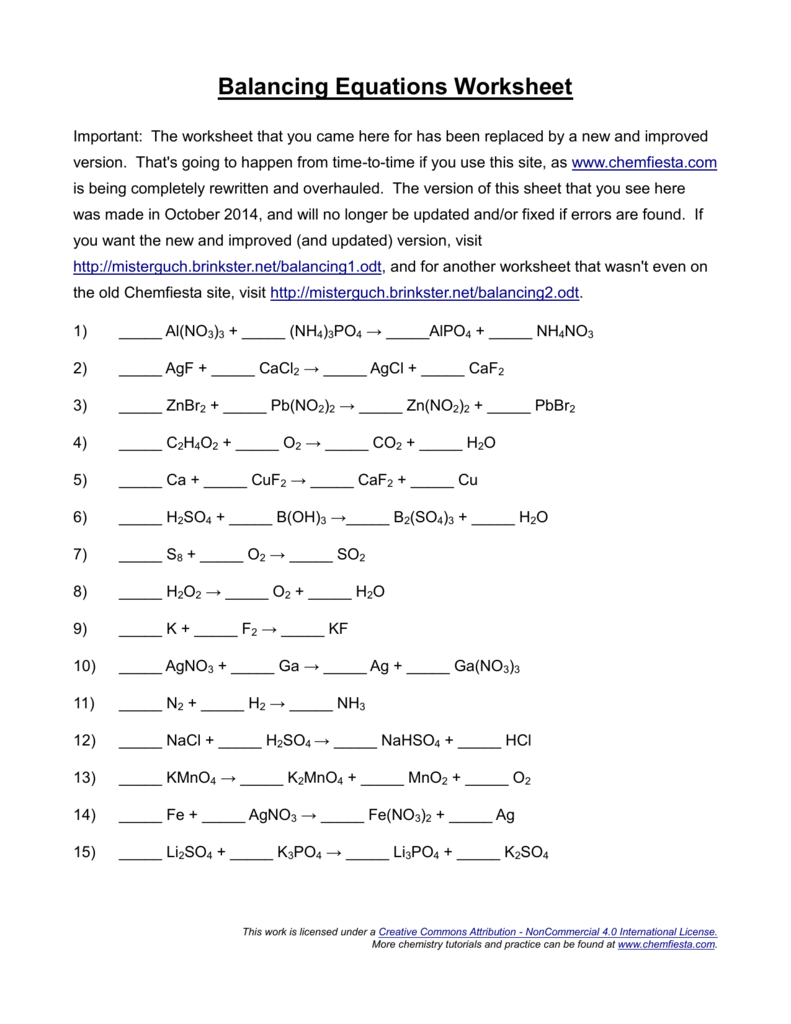
This means that chemical equations need to be balanced before they can be used for calculations. Once you understand how to balance an equation in terms of mass, you're ready to learn how to balance an equation for both mass and. ⚛ reactants are written on the left hand side of the chemical equation. Identify the reactants and the products in the reaction and. Examples of balancing chemical equations. Try each question first, using a pen and paper. How to balance a chemical reaction by making sure you have the same number of atoms of each element on both sides. Avogadro's number, chemical equation, chemical formula, chemical reaction, coefficient, combination, combustion, conservation of matter, decomposition, double replacement, molar mass, mole, molecular mass, molecule, product, reactant. 0 ratings0% found this document useful (0 votes). Always use the upper case for the first character in the element name and the lower case for the. Answers in as fast as 15 minutes. Balance each of the following equations. Enter a chemical equation to balance:
1 the first step in balancing a chemical equation is to count each type of atom in reactants and products. Balance and classify five types of chemical reactions: 2 k3po4 + 3 mgcl2 = 6 kcl + mg3(po4)2 reaction type: (coefcients equal to one (1) do not need to be shown in your answers). It represents how reactants react to give a completely new product.

Balancing chemical equations name hour standard 1 learning module 10 background information and why: Always use the upper case for the first character in the element name and the lower case for the. While balancing the reactions, the number of atoms on each side is presented as visual, histogram, and numerical data. Identify the reactants and the products in the reaction and. Ask expert tutors you can ask you can ask you can ask (will expire ). Write a balanced equation for the chemical reaction below. Try each question first, using a pen and paper. To understand chemistry, students must be confident in representing chemical and physical changes with chemical equations, and this requires lots of practice and exposure. This means that chemical equations need to be balanced before they can be used for calculations. This is a set of worked examples of how to balance chemical equations, a very important skill in chemistry. balancing chemical equations x + yxyintroductiongame. Balance the following chemical equations. Potassium (s) + water (l) potassium hydroxide (aq) + hydrogen (g) answer:
262 Balancing Chemical Equations Answer Key: A balanced chemical equation has an equal number of atoms of different elements in the reactants and products.
Refference: 262 Balancing Chemical Equations Answer Key
0 komentar